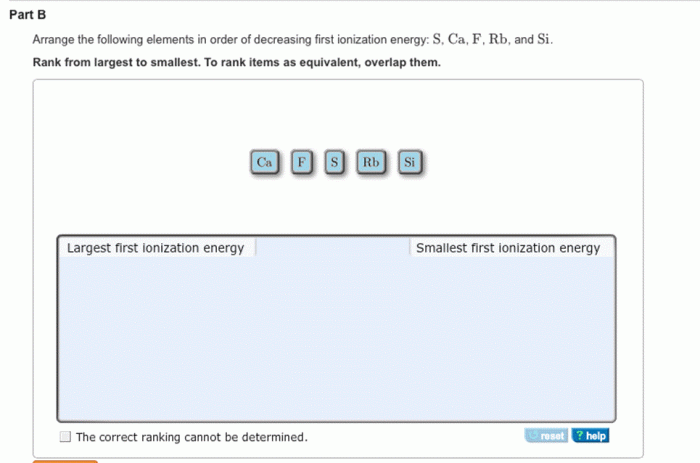
Arrange the elements in decreasing order of first ionization energy. – Ionization energy, a crucial concept in chemistry, plays a pivotal role in determining the chemical properties of elements. This article delves into the fascinating world of ionization energy, exploring the factors that influence it, the methods used to determine it, and its diverse applications across scientific disciplines.
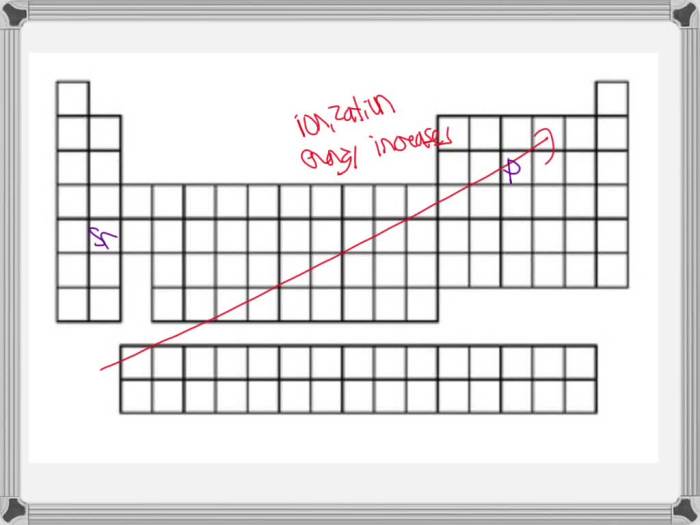
The periodic table, organized based on ionization energy trends, serves as a roadmap for understanding the behavior of elements. As we traverse the periodic table, ionization energy exhibits intriguing patterns, providing insights into atomic structure and chemical reactivity.
Introduction: Arrange The Elements In Decreasing Order Of First Ionization Energy.
Ionization energy is a crucial concept in chemistry that measures the energy required to remove an electron from an atom or ion. Understanding ionization energy provides valuable insights into the chemical behavior and reactivity of elements.
The periodic table, organized based on atomic number and electron configuration, exhibits a distinct pattern in ionization energy. Elements with lower ionization energies are more likely to lose electrons, while those with higher ionization energies tend to retain their electrons.
Factors Affecting Ionization Energy
Atomic Radius
Ionization energy generally decreases with increasing atomic radius. This is because larger atoms have a weaker electrostatic attraction between the nucleus and outermost electrons, making it easier to remove an electron.
Nuclear Charge
Ionization energy increases with increasing nuclear charge. A higher nuclear charge exerts a stronger electrostatic attraction on electrons, making it more difficult to remove them.
Electron Configuration
Elements with stable electron configurations, such as noble gases, have high ionization energies. This is because their electrons are arranged in a way that minimizes electron-electron repulsion and maximizes stability.
Methods for Determining Ionization Energy
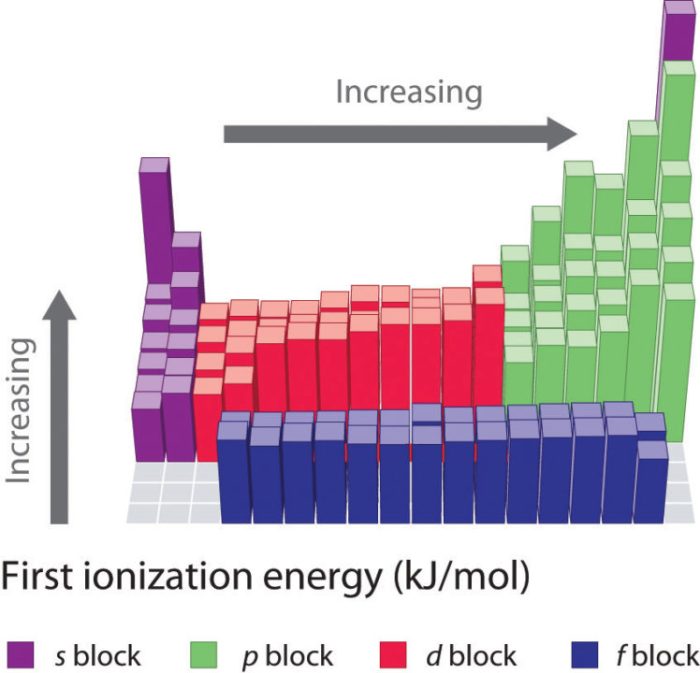
Electron Impact Spectroscopy
This technique bombards gaseous atoms with high-energy electrons. The energy required to remove an electron from the atom is determined by analyzing the energy of the scattered electrons.
Photoionization, Arrange the elements in decreasing order of first ionization energy.
This method uses ultraviolet or X-ray radiation to ionize atoms. The ionization energy is determined by measuring the wavelength of the radiation required to remove an electron.
Applications of Ionization Energy Data
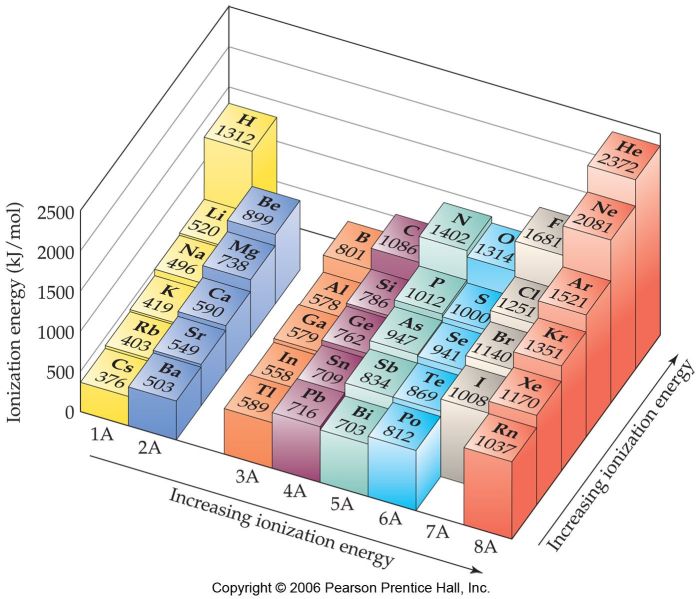
Chemical Bonding
Ionization energy plays a significant role in determining the type of chemical bond formed between atoms. Elements with low ionization energies tend to form ionic bonds, while those with high ionization energies form covalent bonds.
Reactivity
Ionization energy provides insights into the reactivity of elements. Elements with low ionization energies are more reactive, as they can easily lose electrons and form chemical bonds.
Materials Science
Ionization energy data is essential in understanding the properties and behavior of materials. For example, materials with high ionization energies are often used as semiconductors and insulators.
Popular Questions
What is ionization energy?
Ionization energy refers to the energy required to remove an electron from an atom or ion in its gaseous state.
How is ionization energy measured?
Experimental techniques such as electron impact spectroscopy and photoionization are employed to measure ionization energy.
What factors influence ionization energy?
Ionization energy is influenced by factors such as atomic radius, nuclear charge, and electron configuration.