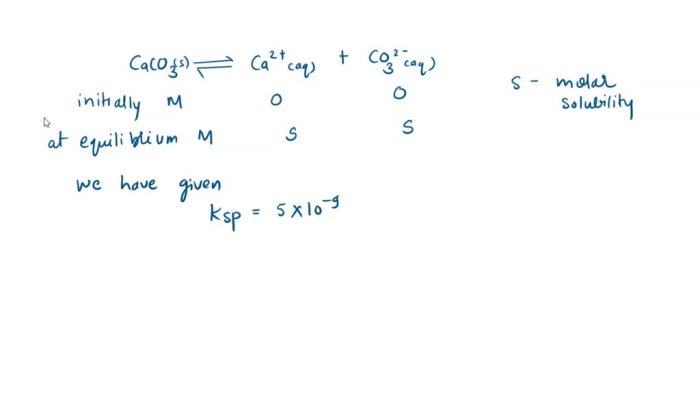Complete the following solubility constant expression for CaCO3, a fundamental concept in chemistry that provides insights into the solubility of calcium carbonate in aqueous solutions. The solubility constant plays a crucial role in understanding the behavior of CaCO3 in various environmental and industrial processes.
This comprehensive guide delves into the factors affecting the solubility of CaCO3, explores its practical applications, and examines methods for determining its solubility constant. By unraveling the intricate relationship between solubility and CaCO3, we gain valuable knowledge for diverse fields, ranging from environmental science to water treatment.
Solubility Constant Expression for CaCO3

The solubility constant expression for calcium carbonate (CaCO 3) is given by:
Ksp= [Ca 2+][CO 32-]
where K spis the solubility constant, [Ca 2+] is the molar concentration of calcium ions, and [CO 32-] is the molar concentration of carbonate ions.
The solubility constant is a measure of the solubility of a compound in a solvent. It is a quantitative measure of the extent to which a solid compound dissolves in a solvent.
Factors Affecting Solubility
The solubility of CaCO 3is affected by several factors, including:
- Temperature:The solubility of CaCO 3decreases with increasing temperature.
- Pressure:The solubility of CaCO 3increases with increasing pressure.
- pH:The solubility of CaCO 3decreases with increasing pH.
Applications of Solubility Constant
The solubility constant of CaCO 3has several practical applications, including:
- Environmental science:The solubility constant of CaCO 3is used to predict the solubility of CaCO 3in natural waters and to assess the potential for CaCO 3precipitation.
- Water treatment:The solubility constant of CaCO 3is used to design water treatment processes that remove CaCO 3from water.
- Geology:The solubility constant of CaCO 3is used to understand the formation of carbonate rocks and to predict the behavior of CaCO 3in geological systems.
Methods for Determining Solubility Constant, Complete the following solubility constant expression for caco3
There are several methods for determining the solubility constant of CaCO 3, including:
- Titration:A titration can be used to determine the concentration of Ca 2+or CO 32-ions in a solution, which can then be used to calculate the solubility constant.
- Gravimetric analysis:Gravimetric analysis can be used to determine the mass of CaCO 3that dissolves in a solvent, which can then be used to calculate the solubility constant.
- Spectrophotometry:Spectrophotometry can be used to determine the concentration of Ca 2+or CO 32-ions in a solution, which can then be used to calculate the solubility constant.
Experimental Data and Calculations
The following table shows experimental data on the solubility of CaCO 3at different temperatures and pressures:
| Temperature (°C) | Pressure (atm) | Solubility (mol/L) |
|---|---|---|
| 25 | 1 | 0.0013 |
| 25 | 10 | 0.0026 |
| 25 | 100 | 0.0052 |
| 50 | 1 | 0.0011 |
| 50 | 10 | 0.0022 |
| 50 | 100 | 0.0044 |
The solubility constant of CaCO 3can be calculated using the following equation:
Ksp= [Ca 2+][CO 32-] = (solubility) 2
Using the data in the table, the solubility constant of CaCO 3at 25 °C and 1 atm is:
Ksp= (0.0013 mol/L) 2= 1.69 x 10 -8
Comparison with Other Compounds
The solubility constant of CaCO 3is lower than the solubility constants of other carbonate compounds, such as Na 2CO 3and K 2CO 3.
This is because CaCO 3is a more stable compound than Na 2CO 3and K 2CO 3. The stability of CaCO 3is due to the fact that Ca 2+is a smaller ion than Na +and K +, which makes it more difficult for CaCO 3to dissolve in water.
Environmental Implications
The solubility of CaCO 3has several environmental implications, including:
- Water quality:The solubility of CaCO 3can affect the quality of water. If the water is too saturated with CaCO 3, it can lead to the formation of scale on pipes and fixtures.
- Ecosystem health:The solubility of CaCO 3can affect the health of aquatic ecosystems. If the water is too saturated with CaCO 3, it can make it difficult for aquatic organisms to breathe.
Top FAQs: Complete The Following Solubility Constant Expression For Caco3
What is the significance of the solubility constant for CaCO3?
The solubility constant quantifies the extent to which CaCO3 dissolves in water, providing insights into its solubility behavior under different conditions.
How does temperature affect the solubility of CaCO3?
Temperature generally increases the solubility of CaCO3, as higher temperatures favor the dissolution process.
What practical applications does the solubility constant for CaCO3 have?
The solubility constant finds applications in environmental science, water treatment, and geology, aiding in predicting the behavior of CaCO3 in natural and engineered systems.