Formulas of ionic compounds worksheet – The realm of ionic compounds and their formulas unfolds in this captivating exploration. We delve into the intricacies of ionic bond formation, unravel the rules governing formula writing, and embark on a hands-on journey with our meticulously crafted worksheet.
Comprehending ionic compounds is not merely an academic pursuit; it holds profound significance in understanding the very fabric of matter and its myriad applications in our daily lives.
Ionic Compounds
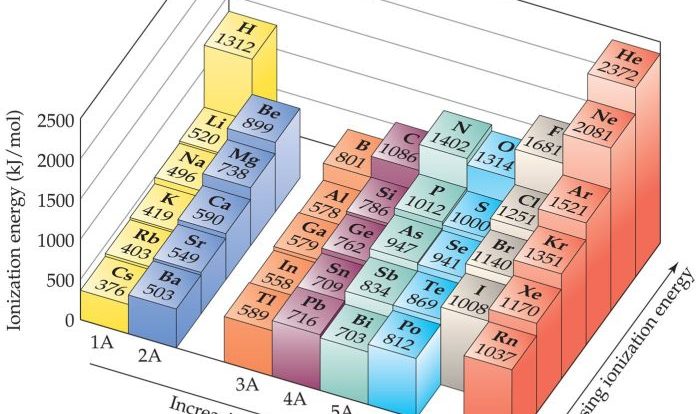
Ionic compounds are chemical compounds composed of ions, which are atoms or molecules that have lost or gained electrons, resulting in a net electric charge. The electrostatic attraction between oppositely charged ions holds the compound together.Ionic bonds are formed when one atom transfers one or more electrons to another atom.
The atom that loses electrons becomes a positively charged ion, called a cation, while the atom that gains electrons becomes a negatively charged ion, called an anion. The oppositely charged ions are attracted to each other by the electrostatic force, forming an ionic bond.Examples
of ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and calcium fluoride (CaF2).
Formulas of Ionic Compounds: Formulas Of Ionic Compounds Worksheet
Ionic compounds are formed by the transfer of electrons from one atom to another, resulting in the formation of positively charged cations and negatively charged anions. To write the formula of an ionic compound, we need to know the charges of the ions involved.
Rules for Writing Formulas of Ionic Compounds
The following rules are used to write formulas of ionic compounds:
- The cation is always written first, followed by the anion.
- The charges of the ions must balance each other out. For example, if the cation has a charge of +2, the anion must have a charge of
2.
- The subscripts in the formula indicate the number of atoms of each element present in the compound. For example, NaCl represents sodium chloride, which contains one atom of sodium and one atom of chlorine.
Variable Charge
Some elements can have variable charges. For example, iron can have a charge of +2 or +3. When writing the formula of an ionic compound containing an element with variable charge, we need to specify the charge of the ion.
Step-by-Step Procedure for Writing Formulas of Ionic Compounds, Formulas of ionic compounds worksheet
- Determine the charges of the ions involved.
- Write the symbol of the cation first, followed by the symbol of the anion.
- Balance the charges of the ions by adjusting the subscripts.
Worksheet Activities
To enhance understanding and reinforce the concepts of ionic compound formulas, it is beneficial to provide students with practice problems.
An effective worksheet should include a variety of problems that cover different levels of difficulty. This allows students to gradually build their skills and confidence in writing ionic compound formulas.
Worksheet Design
The worksheet should be designed to:
- Include a clear set of instructions explaining the task.
- Provide a table or chart where students can write the formulas of the ionic compounds.
- Include a variety of problems that cover different types of ions, including monatomic and polyatomic ions.
- Incorporate problems of varying difficulty levels to accommodate students with different levels of understanding.
- Provide an answer key for students to check their work.
Sample Problems
Sample problems for the worksheet could include:
- Write the formula for sodium chloride.
- Write the formula for calcium oxide.
- Write the formula for potassium sulfate.
- Write the formula for ammonium nitrate.
- Write the formula for magnesium phosphate.
Answer Key
The answer key should provide the correct formula for each problem.
- NaCl
- CaO
- K 2SO 4
- NH 4NO 3
- Mg 3(PO 4) 2
Additional Resources
Expanding your knowledge on ionic compounds and formula writing can be greatly enhanced through the exploration of online resources and videos.
These materials provide valuable insights, interactive simulations, and real-world examples that can deepen your understanding of the subject matter.
Online Resources
- Ionic Compounds: https://www.khanacademy.org/science/chemistry/chemical-bonds/ionic-bonds/a/ionic-compounds
- Formula Writing for Ionic Compounds: https://www.thoughtco.com/how-to-write-formulas-for-ionic-compounds-606637
- Interactive Simulation: https://phet.colorado.edu/sims/html/ionic-compounds/latest/ionic-compounds_en.html
Importance of Understanding Ionic Compounds
Ionic compounds play a crucial role in chemistry, as they are involved in various chemical reactions and processes.
Understanding their properties, behavior, and formula writing is essential for comprehending the fundamentals of chemistry and its applications.
Applications of Ionic Compounds in Everyday Life
- Table Salt (NaCl):A common ionic compound used for seasoning and preserving food.
- Baking Soda (NaHCO3): Used as a leavening agent in baking and as an antacid.
- Calcium Chloride (CaCl2): Used as a deicing agent on roads and as a fertilizer.
- Iodine (KI):Used as a disinfectant and in the production of iodized salt.
FAQ
What are ionic compounds?
Ionic compounds are formed when a metal loses one or more electrons to a non-metal, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions) that are held together by electrostatic attraction.
How do I write the formula of an ionic compound?
To write the formula of an ionic compound, you must balance the charges of the ions involved. The cation is written first, followed by the anion, and subscripts are used to indicate the number of each ion needed to achieve charge neutrality.